Columns
Japan Society of Quality Assurance and Academia
 I would like to introduce our organization,
I would like to introduce our organization, ![]() the Japan Society of Quality Assurance (JSQA), in order to promote mutual understanding and collaborations with researchers in academia.
the Japan Society of Quality Assurance (JSQA), in order to promote mutual understanding and collaborations with researchers in academia.
First, let me explain the meaning of "QA" in the name of our organization. QA stands for Quality Assurance. Pharmaceutical products used in medical settings are approved by government through evaluating their efficacy and safety, and manufactured while maintaining a certain level of quality. To carrying out the series of the above-mentioned evaluations properly, Good Practices (GxP) have been legislated to guarantee the reliability of test data and products. JSQA is a group of experts who engage in overseeing QA operations that make final confirmation whether the GxP is properly complied with.
Historically speaking, Good Manufacturing Practice (GMP) was first established, followed by Good Laboratory Practice (GLP), and then, Good Clinical Practice (GCP), Good Quality Practice (GQP), Good Vigilance Practice (GVP), and Good Post-Marketing Study Practice (GPSP) were established. In addition to pharmaceutical products, medical products and cosmetics are subject to Pharmaceutical and Medical Device Act (PMD Act), agricultural chemicals are subject to Agricultural Chemicals Regulation Act, and chemical substances ae subject to Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture. They are all similar regulations and these QA are within the scope of JSQA.
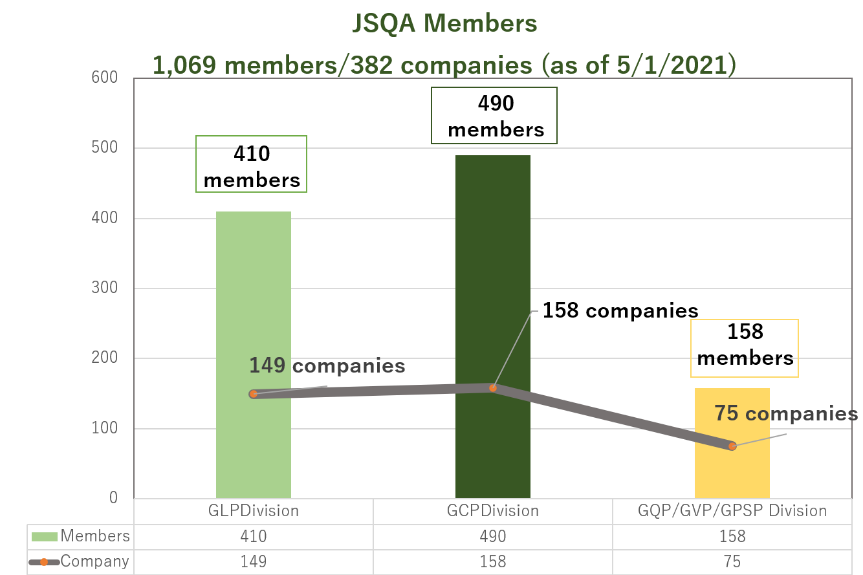
JSQA consists of three divisions, which are GLP, GCP, and GQP/GVP/GPSP, and the members join a division they prefer and work on the study themes for two-year term (current 15th term will end at the end of March 2022). The study themes within a division or common through all divisions are set by each division, their activities are presented at a workshop and summarized in documents at the end of each term, and internally accumulated. The management policies are decided at the general meeting and the specific operation plans are discussed at the steering committee.
The characteristics of the JSQA activities are the following four points.
- QA professionals can participate in JSQA regardless of the targe products, target areas or business categories, and share and update their QA ethics and knowledge in an environment that transcend beyond companies and business types.
- QA professionals oversee studies and manufacture from beginning to end, not merely checking the final data or product quality. From the quality maintenance viewpoint, QA professionals advise to improve work contents as necessary, therefore, they involve in trainings for employees and related personnel to raise awareness of GxP and examine and ensure the reliability when introducing new technologies. For this reason, research activities of JSQA are vary from creation of training materials for GxP to acquisition of a wide range of information including legal regulations and science technology.
- JSQA and regulatory authorities should have similar judging criterion since they are in the same position regarding assuring quality of studies and manufacture. Therefore, JSQA exchanges opinions frequently with Ministry of Health, Labour and Welfare and Ministry of Agriculture, Forestry and Fisheries of Japan.
 GxP are international regulations. Therefore, members from each division have been participated in the annual meetings by QA organizations in the US and UK (they act globally) to obtain information and introduce Japanese situations. In addition, QA organizations in the US, UK and Japan hold a conference in every three years on a rotating basis. Last year (in 2020), Japan hosted the annual conference at Sendai. JSQA also organizes Asian QA Forum in every 2 years and has participated in the international activities proactively.
GxP are international regulations. Therefore, members from each division have been participated in the annual meetings by QA organizations in the US and UK (they act globally) to obtain information and introduce Japanese situations. In addition, QA organizations in the US, UK and Japan hold a conference in every three years on a rotating basis. Last year (in 2020), Japan hosted the annual conference at Sendai. JSQA also organizes Asian QA Forum in every 2 years and has participated in the international activities proactively.
Since our activities originally focus on QA activities in corporations, membership of JSQA is a company basis, and individual membership is limited to people who want to continue to participate in JSQA after their retirement. However, in recent years, the relationship between Academia and JSQA has become more stronger due to the following reasons; medical institutions are joined the clinical trial institutions that apply GCP, GCP activities at medical institutions, such as the establishment of site management offices, have become more vigorous, investigator-initiated clinical trials and practical applications of the results of basic research gain momentum, and clinical research has become more oriented to ensure reliability comparable to GCP. In such a changing environment, if people in academia wants to join our activities, we accept them as individual members.
At the end, let me introduce some examples of our current activities related to academia.
- JSQA actively participates in academic meetings, holding seminars, and presenting outcomes. GLP Division and GCP Division held seminars and made poster presentations respectively at The Japanese Society of Toxicology and Conference on CRC and Clinical Trials.
- JSQA prepares and provides educational materials for professionals who in charge of clinical trials.
- JSQA examines countermeasures for problems occurring in clinical trials at medical institutions.
- JSQA provides inspection materials for clinical trials using clinical research methods.
- JSQA considers measures to ensure the reliability of scientific research regarding research misconduct and provides the information to universities, etc.
- JSQA invites experts belonging to universities and provide lectures, including special lectures at regular meetings and keynote sessions at international QA conferences.
 If you are interested in our activities, please visit our website at https://www.jsqa.com/en/, and drop in at our exhibition booth at academic conferences. Also, please contact us if you would like to join us.
If you are interested in our activities, please visit our website at https://www.jsqa.com/en/, and drop in at our exhibition booth at academic conferences. Also, please contact us if you would like to join us.
June 2021
Yoshinobu Hirayama
President
Japan Society of Quality Assurance