Columns
Biotech Engineering Company, Striving for Value Creation - For Comprehensive Healthcare System for Children as well as Families, and Society -
![]() Kidswell Bio Corporation, with the corporate philosophy of "Biotech Engineering Company, Striving for Value Creation - For Comprehensive Healthcare System for Children as well as Families and Society -," works on R&D activities on a day-to-day basis to provide innovative pharmaceuticals and therapeutics for patients suffering from diseases, especially children, at an early stage and to contribute to the realization of a society where everyone can live in a happy and bright life.
Kidswell Bio Corporation, with the corporate philosophy of "Biotech Engineering Company, Striving for Value Creation - For Comprehensive Healthcare System for Children as well as Families and Society -," works on R&D activities on a day-to-day basis to provide innovative pharmaceuticals and therapeutics for patients suffering from diseases, especially children, at an early stage and to contribute to the realization of a society where everyone can live in a happy and bright life.
In 2001, we established as Gene Techno Science to develop the results of research at the Hokkaido University Institute for Genetic Medicine involving functions of immune proteins. In July 2021, we made a new start as Kidswell Bio Corporation with the management vision of "Kids Well, All Well", contributing to the health and well-being of all stakeholders surrounding children.
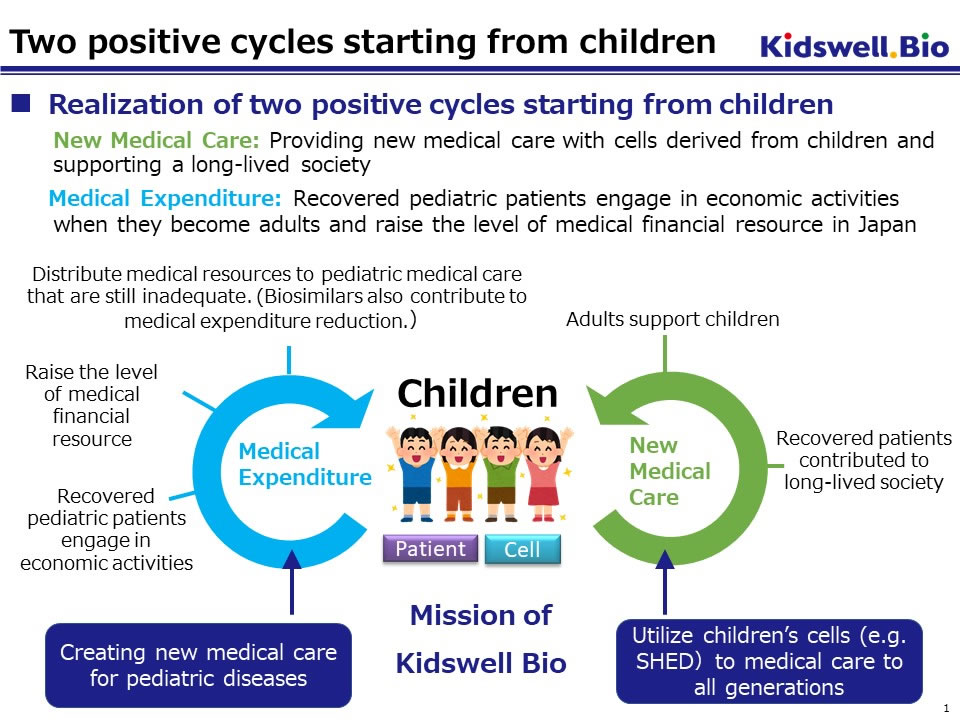
Toward improving access to treatment and contributing to the maintenance of our national social security system, we have already launched three biosimilar products with partner pharmaceutical companies. In addition, we have been actively engaged in the R&D of regenerative medicine utilizing stem cells from human exfoliated deciduous teeth (SHED) and new bio (an antibody) drugs by leveraging profits from biosimilars business. Through creation of innovative pharmaceuticals and therapeutic treatments, we would like to support people recovering from diseases to return to economic activities and contribute to raising our national healthcare financial resources.
S-QuatreSM - Tooth to Life-Changing Medicine -
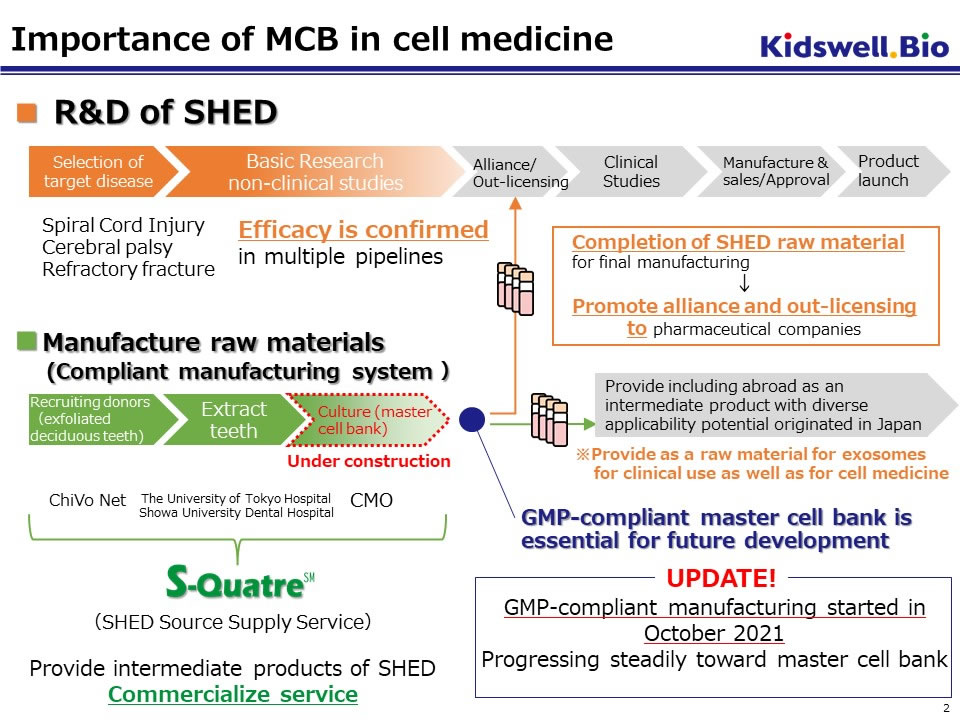
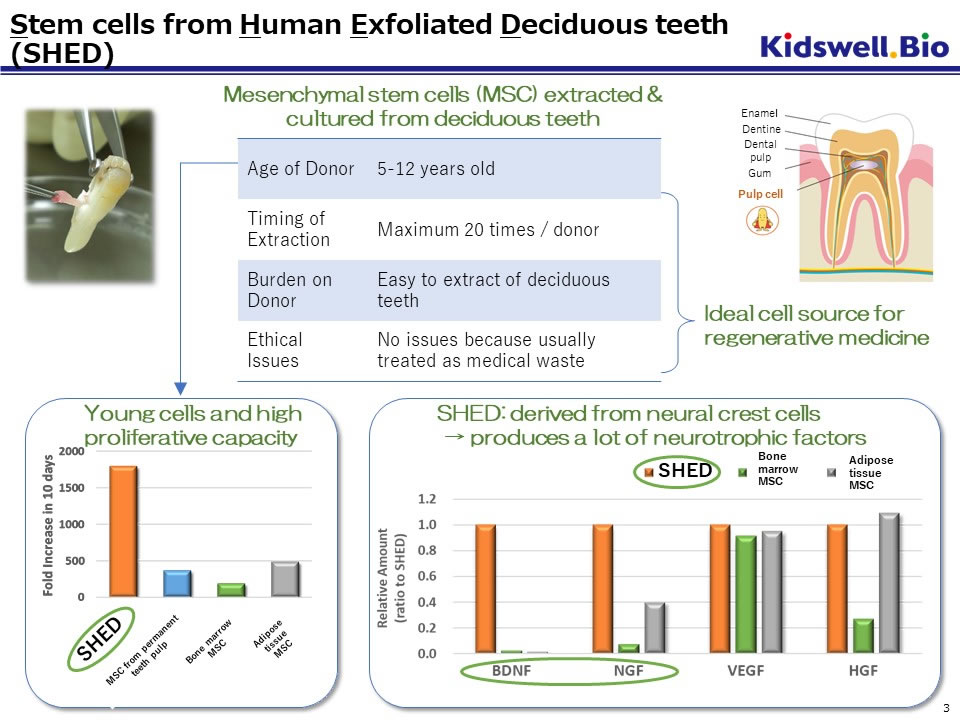
SHED, which we have been developing as a raw material for regenerative medicine, is a mesenchymal stem cell (MSC) extracted from a dental pulp cavity inside exfoliated deciduous teeth and is easy to differentiate into bone and nerve cells. Especially SHEDs from young donors have shown higher proliferative activity and secretory capacity of various growth factors (particularly neurotrophic factors) compared to stem cells from other tissues. The efficacy of SHED has been confirmed in multiple animal models such as spinal cord injury, cerebral palsy, and bone injury. The master cell bank (MCB) of SHED for clinical application is nearly completed.
We acquire deciduous teeth from domestic donor children in an ethically appropriate manner and started manufacturing master cell bank (MCB) in compliance with GMP. Since the GMP-compliant MCB manufacturing system has been steadily progressing, we have released S-QuatreSM, a service that manufactures and provides safety-assured homogeneous SHED for clinical use.
S-QuatreSM offers a one-stop service to providing highly reliable and safe domestically produced SHED as an intermediate product for regenerative medicine through business collaboration, which we have established as a core company, with donor-recruiting institutions, medical institutions, manufacturing storage companies, and transportation companies.
We will continue to strive for R&D of SHED more actively and lead to practical application as a regenerative medicine by instilling S-QuatreSM to academia and companies interested in regenerative medicine research.
March, 2022
Shinya Kurebayashi
Corporate Officer, CBO, Business Development Div.
Kidswell Bio Corporation