Columns
Future Challenges of Drug Discovery Research against Antimicrobial Resistance (AMR)
Currently, international measures against Antimicrobial Resistance (AMR) are an urgent issue. Importance of Development of antimicrobial agents with new mechanisms is recognized by the vigorous activities by WHO and CDC and various supports for drug discovery research and development have become active worldwide, however, they are not yet fully operated.
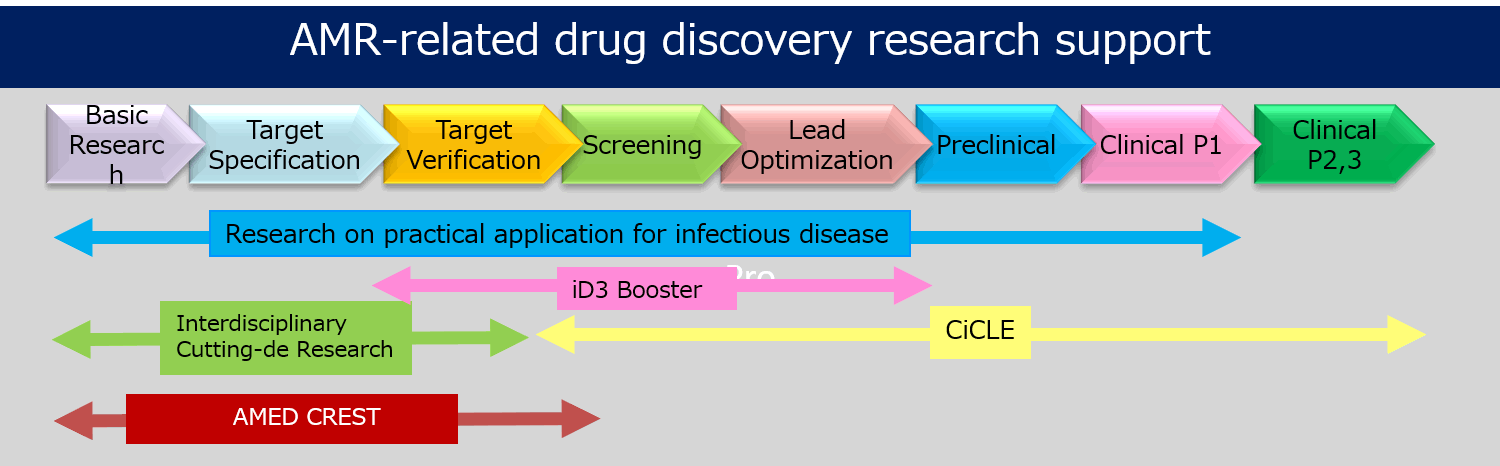
Under these circumstances, the Japanese Agency for Medical Research and Development (AMED) plays a part in promoting R&D in Japan including new vaccines, rapid diagnostics, and antimicrobial agents with new mechanisms, which are needed to prevent AMR in the future. Especially, AMED supports the following 5 projects in order to promote AMR-related drug discovery research and development.
1) Comprehensive Drug Discovery Support Project (Drug Discovery Booster)
2) Research Project to Promote Development of Innovative Drugs for Emerging and Reemerging Infectious Diseases
3) Multidisciplinary Research Area for Foundation Development
4) Medical R&D Innovation Platform Creation Project (CiCLE)
5) Innovative Advanced R&D Support Project (AMED-CREST)* 1) & 2) are supervised by the Division of Strategic Planning and Evaluation, Department of Innovative Drug Discovery and Development. 3), 4), and 5) are supervised by the Division of Basic Medical Research, Department of Basic Medical Research, Division of Planning and Coordination, Department of Cyclic Innovation, and Coordination, Division of Innovative Research and Development, Department of Innovation and Clinical Research Center, respectively.
To apply the results generated from these AMED support projects into practice, efforts are required not only with business perspective, but also with a social contribution perspective that takes into account the particularities of infectious diseases as well. Therefore, it becomes a key to promote research and development in collaboration with pharmaceutical companies, research institutions including universities, academic societies, and regulatory authorities.
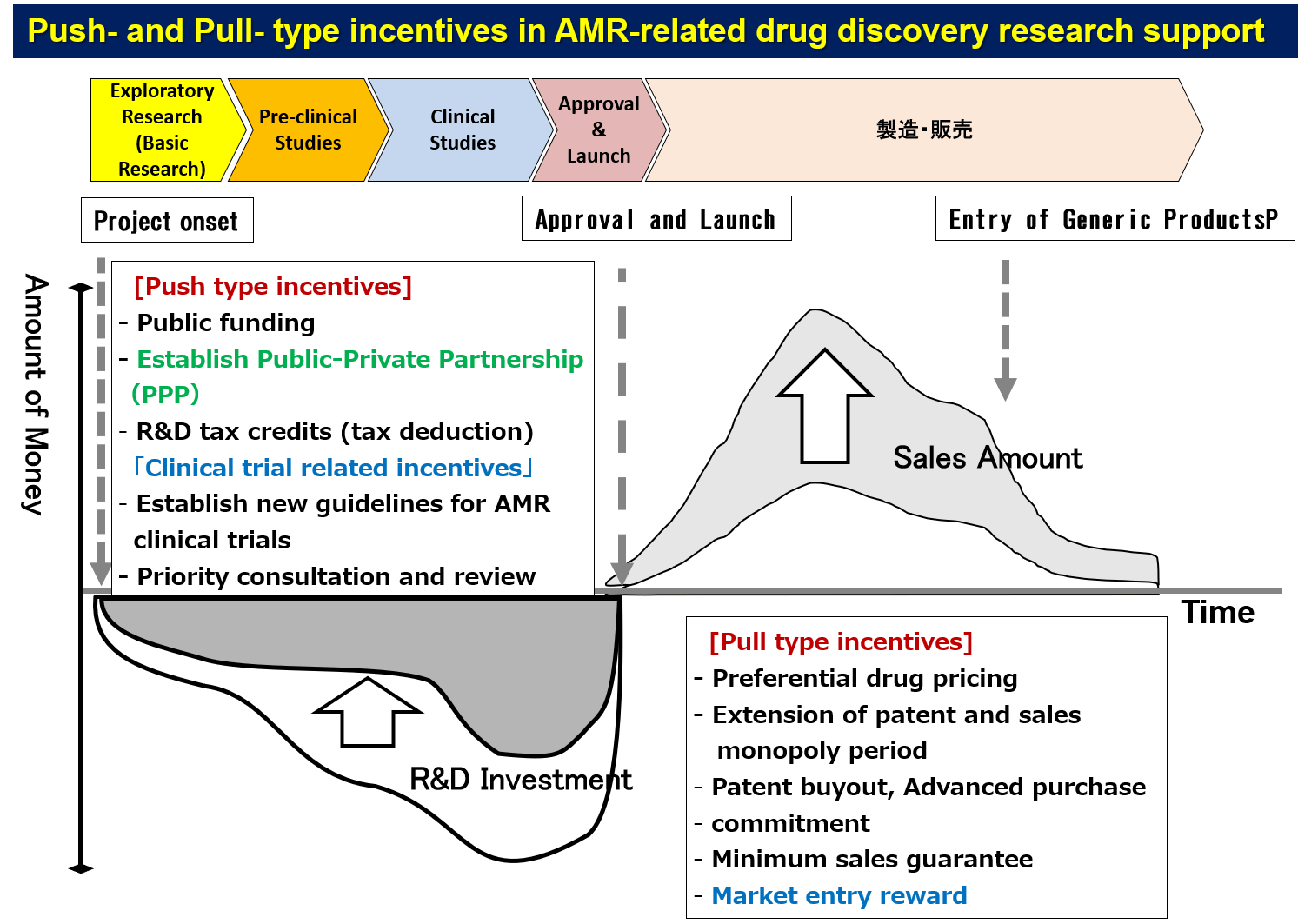
To achieve the goal, a mechanism is required to effectively connect academia, industry, and regulatory authorities to induce active cooperation and stimulate R&D activation for infectious diseases that are not high prioritized from a business perspective. So-called push incentives (support for R&D funding, etc.) and pull incentives (advanced purchase commitments, market-and demand-forming support, etc.) are now becoming indispensable as R&D engines.
For Push incentives, the IMI (Innovative Medicines Incentives) in Europe, CARB-X (Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator), and BARDA (Biomedical Advanced Research and Development Authority) in the United States provide detailed support with large-scale budgets and getting positive outcomes. In addition, legislation is being established on extension of patent period and data protection period, subsidy system of research and development expenditure, and taxation reduction measures are also made. The platforms which promote the R&D of the antimicrobial agent are being established.
Even if such incentives are developed, as long as pharmaceutical companies regard opportunities for drug discovery of infectious diseases from a conventional business perspective, there is no assurance that they will return to drug discovery of infectious diseases. Therefore, in addition to these platforms, I personally believe that new ideas, such as building a sustainable R&D system that incorporates concepts of sustainable Development Goals (SDGs) will be necessary. Therefore, it will become very important to establish a new business model that effectively links each stakeholder of industry, academia, and government considering the particularities of infectious diseases (such as crisis management at the global level.)
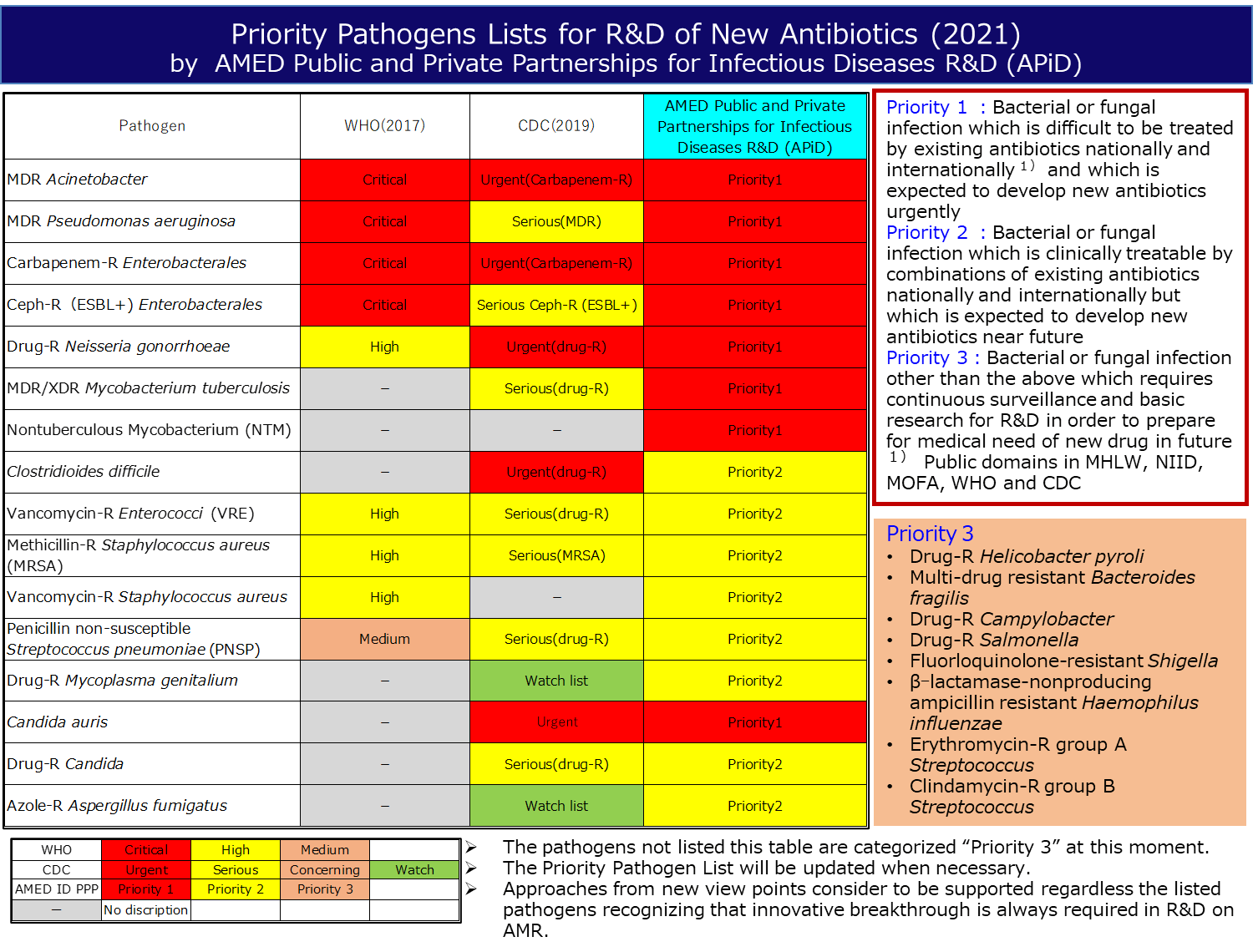
In view of this situation, we opened "![]() AMED Public and Private Partnerships for Infectious Diseases R&D" web page in AMED iD3 Catalyst Unit's website. Prior to this, AMED Public and Private Partnerships for Infectious Diseases R&D has prepared a list of pathogens with priority level of urgency of new antibiotic development in cooperation with industry, academia, last year with the aim of activating AMR-based antibiotic discovery research. We posted the list on the web page. If you are interested in infectious disease drug discovery, we appreciate your visit.
AMED Public and Private Partnerships for Infectious Diseases R&D" web page in AMED iD3 Catalyst Unit's website. Prior to this, AMED Public and Private Partnerships for Infectious Diseases R&D has prepared a list of pathogens with priority level of urgency of new antibiotic development in cooperation with industry, academia, last year with the aim of activating AMR-based antibiotic discovery research. We posted the list on the web page. If you are interested in infectious disease drug discovery, we appreciate your visit.
I sincerely hope that the HP will be utilized, and AMR-related drug discovery research will be activated and accelerated.
We appreciate your cooperation and support in AMR drug discovery research!
Akihiko Fujie
Secretariat
AMED Public and Private Partnerships for Infectious Diseases R&D